Medical Disclaimer: The following content should not be used as medical advice or as a recommendation for any specific supplement or medication. It is important to consult your health care provider prior to starting a new medication or altering your current dosage.
SnoreRx Mouthpiece
The SnoreRx Mouthpiece features a highly customizable design. The boil-and-bite template adapts to your teeth, while a user-friendly tab allows you to adjust the lower jaw position in 1-millimeter increments.
Snoring occurs when a person’s airway narrows during sleep, partially obstructing the flow of air and causing soft tissues to vibrate. The noise can disrupt sleep for a bed partner and sometimes awaken the person snoring.
Many people find that anti-snoring mouthpieces are a simple, affordable way to stop snoring. Mandibular advancement devices (MADs) push the lower jaw forward to keep the airway open, while tongue-retaining devices (TRDs) anchor the tongue to prevent it from slipping back.
We’ll explore the best anti-snoring mouthpieces and mouthguards and discuss how to know if an anti-snoring device is right for you.
Looking for more snoring solutions? See our guide to the best mattresses for snoring. You may also find relief with one of the best pillows for snoring.
Sleep Doctor’s Picks
Best Overall
SnoreRx Mouthpiece
Details
Mouthpiece Type: Mandibular advancement device (MAD)
Price: $60
Pros
- Users can make ongoing adjustments to jaw positioning
- Easy-to-use boil-and-bite design
- Competitive price-point
Cons
- Only comes in one size
- No warranty
- Some users may experience jaw or dental discomfort, especially at first
The SnoreRx checks a lot of boxes for people in search of a comfortable anti-snoring mouthguard. The device is easy to customize, fully adjustable, conducive to mouth breathing, and affordable.
Why We Like It
For added comfort, the SnoreRx is soft, pliable, and lightweight. Small ventilation holes promote airflow while you sleep, allowing you to breathe through your mouth without obstruction. The device is adjustable in increments of 1 millimeters up to 6 millimeters, allowing for full lateral movement. This is a major perk for anyone who has found other mouthpieces too restrictive.
How Does It Work?
The SnoreRx Mouthpiece is a fully customizable mandibular advancement device designed to curb snoring by repositioning your jaw. The boil-and-bite design allows you to easily make an impression that’s custom-fitted for your mouth. After the impression has been made, you can adjust how far the guard pushes your jaw forward.
Customizing the SnoreRx is an easy, straightforward process. Bring a glass of water to a boil in a microwave, and then dip the SnoreRx in the glass for exactly two minutes. Then remove the mouthguard and immediately dip it in a glass of cold water. Wait a few seconds, then take the mouthguard out of the water, place it in your mouth, and bite down for three minutes. Lastly, remove the device from your mouth and soak it in the glass of cold water for 10 minutes to allow it to set. Don’t worry if the impression isn’t a perfect fit after the first setting — you can attempt a second impression if needed.
Best Value
ZQuiet Anti-Snoring Mouthpiece
Details
Mouthpiece Type: Mandibular advancement device (MAD)
Price: $70
Pros
- Universal design with no need for custom molding
- Comes with two sizes, for 2- or 6-millimeter jaw advancement
- Flexible design allows mouth opening and side-to-side mobility
Cons
- No custom fit option
- Larger size is more effective for heavy snoring, but may cause tooth and jaw pain
- Somewhat bulky design may cause excessive salivation
The ZQuiet Anti-Snoring Mouthpiece is easier to use than most competing MADs. Two sizes and a lengthy trial period help ensure you’ll find a comfortable fit and effective advancement.
Why We Like It
The mouthpiece is a hassle-free alternative to customizable boil-and-bite MADs. Designed for a universal fit without any molding whatsoever, virtually anyone can use the ZQuiet as long as they select the correct size. The flexible design promotes side-to-side jaw movements and allows you to comfortably breathe through your mouth. ZQuiet recommends trying the smaller device first. If your snoring persists, you can transition to the larger mouthguard.
How Does It Work?
The ZQuiet is available in two sizes. If you need minor advancement from your mouthguard, we recommend the smaller device that advances your jaw 2 millimeters. The larger mouthguard with an advancement of 6 millimeters may be more suitable if you have a larger jaw. Your purchase will include one of each, allowing you to test both to find the best fit.
You should clean and sanitize the device each morning. For a small upcharge, you can add a bottle of ZQuiet Clean to your purchase. You can order single mouthguards whenever your original device needs to be replaced. The ZQuiet Clean solution is also available for individual purchase.
Best Adjustable
VitalSleep Anti-Snoring Mouthpiece
Details
Mouthpiece Type: Mandibular advancement device (MAD)
Price: $70
Pros
- Up to 8-millimeter lower jaw advancement using included tool
- Two sizes available
- Small opening at the front to allow mouth breathing
Cons
- Fitting process may take several tries
- Some sharp parts
- May not fit larger mouths
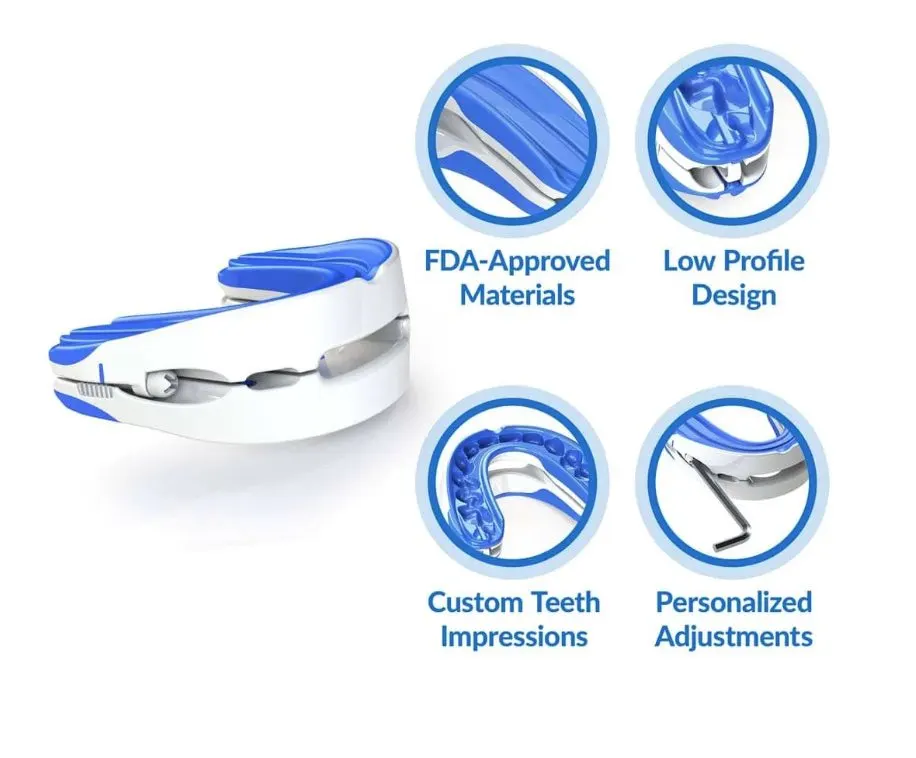
The VitalSleep Anti-Snoring Mouthpiece is a highly adjustable MAD that should benefit even the heaviest of snorers. A customizable boil-and-bite design, two size options, and advancement of up to 8 millimeters help ensure most people can comfortably use the device.
Why We Like It
The VitalSleep Anti-Snoring Mouthpiece offers a level of adjustability that is hard to find with MADs sold today. Using the adjustment tool included with your order, you can slide the device’s lower tray forward or backward in 1 millimeter increments up to 8 millimeters. This ensures people who need minor, moderate, or even major jaw advancement can improve their airflow and reduce snoring with the mouthguard.
How Does It Work?
You can choose from two sizes for your VitalSleep device, one of which is roughly 10% larger than the other. To make an impression, place the mouthguard in heated water for 45 seconds, then dip it in cold water for three seconds and sink your teeth into the upper and lower trays. On the first night, try the mouthguard in its neutral setting without adjusting the lower tray. Sliding it forward by 1 millimeter each night can help you reach your ideal advancement level in a matter of days.
Best Tongue Stabilizer
Good Morning Snore Solution
Details
Mouthpiece Type: Tongue retaining device (TRD)
Price: $100
Pros
- Simple suction holds tongue forward with no need for custom fitting
- Doesn’t affect teeth or jaw placement, so it’s less likely to cause morning pain
- Minimalist design works with dentures
Cons
- Limits mouth movement
- Tongue may not stay attached when the device is wet
- May cause friction against gums
The Good Morning Snore Solution’s unobtrusive design is ideal for anyone who can’t use MADs due to their medical or dental history. Easy application and a lightweight design make the device a hassle-free alternative to traditional mouthguards.
Why We Like It
The Good Morning Snore Solution is optimal for sleepers who cannot use MADs. These include people with medical conditions such as temporomandibular disorders (TMDs) and advanced gum disease, as well as those with dentures, braces, and dental implants. No boiling or molding is needed, as this mouthguard offers a universal fit.
Good Morning Snore Solution frequently offers promotions that include two or even four mouthguards for the price of one, allowing you to save a lot of money on your purchase.
How Does It Work?
Applying the Good Morning Snore Solution is a quick and painless process. Simply place the device’s flanges between your lips and teeth or dentures, squeeze the bulb between your fingers while inhaling, and insert your tongue into your bulb. The pliable, pear-shaped device uses gentle suction to pull the tongue forward to open up your breathing passages. You may need a few nights to get used to swallowing with the device in place. In the morning, squeeze the bulb and your tongue should slide out easily.
Best Fit
SleepTight Mouthpiece
Details
Mouthpiece Type: MAD/TRD combination device
Price: $80
Pros
- Boil-and-bite customization for a personalized fit
- Hybrid design allows tongue to be held in place using front opening
- Front opening allows mouth breathing when tongue isn’t held in place
Cons
- Fairly bulky design may feel uncomfortable for people with a smaller mouth or jaw
- No mechanisms to adjust the angle of the lower jaw
Most anti-snoring mouthguards are either MADs or TRDs, but the SleepTight incorporates both technologies to ensure a comfortable fit.
Why We Like It
Combining the jaw advancement of a MAD with the tongue retention of a TRD, SleepTight is the best of both worlds for people who snore and have found other mouthguards ineffective. The mouthguard is aerated with a hole in the front, allowing users to comfortably breathe through their mouth. The opening can also serve as a tongue-retaining mechanism for people without nasal obstructions who can breathe through their nose effectively.
How Does It Work?
The device advances the jaw to open up your airway while holding the tongue in place to prevent further obstruction. A boil-and-bite design allows you to create a customized impression for your teeth, and the device can be adjusted later on if you need additional advancement. To make your initial impression, simply submerge the mouthguard in recently boiled water for about 2 minutes and 45 seconds, then bite into the trays after checking to make sure the device is not too hot. To adjust the initial fit, follow the steps for your first impression but move your jaw slightly forward.
Best Device
Smart Nora
Details
-
Price: $359
Pros
- Noninvasive design may appeal to those who prefer not to wear a mouthpiece
- Compatible with most pillow types and all sleeping positions
- Customizable sensitivity and inflation settings
Cons
- Higher price-point compared with over-the-counter mouthguards
- Requires batteries and cables to operate
- Device may have trouble discerning which of two sleeping partners is snoring
Anti-snoring mouthguards have proven to be effective for many sleepers, but some find them uncomfortable and restrictive. If you fall into the latter group, then you’re a prime candidate for the Smart Nora.
Why We Like It
The Smart Nora is virtually silent and does not require a mouthguard of any kind. The device is also compatible with side, back, or stomach sleeping. Additionally, you can adjust the Pebble’s sensitivity level to detect light and heavy snoring. Each component is lightweight and compact, so the Smart Nora doubles as a travel-friendly anti-snoring aid.
The Smart Nora’s expensive price-point reflects its cutting-edge design, but this device may be a solid investment if you are losing sleep from snoring and mouthguards haven’t been helpful.
How Does It Work?
This innovative device consists of three components. First there’s the Pebble, a small device resembling a computer mouse that rests on your nightstand and monitors your breathing patterns using smart technology. You’ll also receive an insert for your pillow and an air pump that rests beside your bed on the floor. If the Pebble detects snoring, the pump will inflate the insert to elevate your head and open up your breathing passages.
Is an Anti-Snoring Mouthpiece Right for You?
An anti-snoring mouthpiece might be right for you if you experience simple snoring with no other symptoms. These devices are generally affordable and easy to source. Additionally, anti-snoring mouthpieces are often available without a prescription.
Anti-snoring devices do come with some potential side effects, most notably drooling and jaw discomfort as you adjust to the mouthpiece. If these side effects bother you, you may prefer other methods to reduce snoring.
You may not be able to wear an anti-snoring mouthpiece if you’ve had recent dental work, if you’re missing teeth, if you have a large overbite, or if you wear braces, a retainer, or dentures. Conditions such as asthma, central sleep apnea, periodontal disease, or chronic jaw pain may also make it unwise to use an anti-snoring mouthpiece.
How Do They Work?
The sound of snoring occurs when muscles relax during sleep and allow soft tissues to block the airway, causing audible vibrations as air works to pass through the smaller space. Anti-snoring mouthpieces widen the airway by either forcing the lower jaw forward or holding the tongue in place.
Types of Anti-Snoring Mouthpieces
The two primary types of anti-snoring mouthpieces are mandibular advancement devices and tongue-retaining devices.
Mandibular Advancement Devices (MADs)
Snoring is often triggered by the lower jaw, or mandible, slipping backward during sleep. Mandibular advancement devices work by bringing the lower jaw forward to keep the airway open. These devices typically use a boil-and-bite process to mold the device to your teeth. MADs may be especially effective if your snoring is at its worst when lying on your back.
MADs can be uncomfortable at first. Some users may experience dry mouth, drooling, or tooth and gum pain. However, many people find that these side effects improve after several weeks.
Tongue-Retaining Devices (TRDs)
Tongue-retaining devices work to keep the airway open by either holding the tongue down with prongs or pulling it forward using gentle suction to prevent it from falling back and blocking the throat.
TRDs are less popular than MADs, but some people prefer TRDs because they don’t exert as much pressure on the teeth and jawbone. As with many MADs, mouth movements may be limited when wearing a TRD. Some users may experience soreness in the tongue, teeth, or gums when they first start using the device.
Video: How You Can Stop Snoring
Watch our video to learn more about snoring, what causes it, and tips for stopping it.
How to Choose an Anti-Snoring Mouthpiece
Anti-snoring mouthpieces from different manufacturers may look similar, but subtle differences in design and materials can impact how comfortable and effective they are.
Adjustability and Customization
Most MADs can be customized to fit your teeth using the boil-and-bite method, similar to a sports mouthguard. Some models also allow you to slide the lower jaw forward in small increments until you find a position that keeps your airway open without feeling too uncomfortable. It may take a few days to adjust to each change.
TRDs are less often customizable, although they may come in different sizes.
Comfort
It can feel uncomfortable to wear a mouthpiece, especially if it’s bulky or if the edges or screws chafe against the inside of your mouth. You can reduce discomfort by selecting a model made with soft materials in a size that fits comfortably inside your mouth.
It may help to start by wearing your new device for short periods during the day. After the initial adjustment period, most sleepers grow accustomed to the feel of the mouthguard and no longer experience significant side effects.
Some, but not all, mouthpieces leave a small opening in case you need to breathe through your mouth. You may find it helpful to use a mouth lubricant if your mouthguard prevents you from fully closing your mouth during sleep. If you have trouble with the mouthpiece falling out, try using a chin strap.
Quality
MADs and TRDs are typically made of medical-grade thermoplastic, which can be molded to eliminate sharp edges and fit the shape of your mouth. This material is resistant to wear and tear, and the best anti-snoring mouthguards are reinforced with extra layers or extra-strong hinges to hold up better against jaw clenching and tooth grinding.
Tongue-retaining devices may be made of softer materials like silicone. Manufacturers usually take care to avoid harmful materials like bisphenol A (BPA) or allergens such as latex, but it’s a good idea to verify the materials before purchasing.
Ease of Cleaning
Anti-snoring mouthpieces need daily cleaning. Most devices can be cleaned with a toothbrush, toothpaste, and warm water. If ease of maintenance is an important factor for you, bear in mind that models with hinges or other extra parts may be more difficult to keep clean.
Pricing
The average cost of an over-the-counter MAD or TRD lies between $50 and $100, with some manufacturers offering subscription plans at a discounted price. Higher-priced models may offer more customization options or better-quality materials. Expect to pay much more for a custom-fitted, medical-grade mouthpiece from a dentist.
Prescription Requirements and Insurance Coverage
Anti-snoring mouthpieces are widely available online and in stores with no need for a prescription. You may also be able to obtain a mouthpiece through your dentist or doctor.
Over-the-counter mouthpieces used for snoring generally do not qualify for insurance coverage, although you may be able to use a flexible spending account or health savings account to defray the cost.
Medicare offers coverage for certain oral appliances if they are used to treat obstructive sleep apnea (OSA), in which case you’ll need a prescription and you’ll need to fulfill the Medicare requirements.
Trial and Warranty
Manufacturers may offer a short trial period, typically between 30 and 60 nights, so you can try out the mouthpiece and return it for a refund if it doesn’t work for you. Since anti-snoring mouthpieces fit each person differently, this trial period can be useful when deciding between models. Keep in mind that you may not qualify for the trial if you buy through a third-party seller.
Mouthpieces may also be covered by a warranty against defects in materials and workmanship.
Frequently Asked Questions
Limited research suggests that anti-snoring mouthpieces can effectively reduce snoring for many people. However, since the reasons for snoring vary from person to person, you may find that some trial and error is needed before finding a solution that works for you.
Anti-snoring mouthpieces are generally considered safe for use by people over 18 years old. They’re designed to be too big to swallow or choke on, and they don’t pose any direct danger to the upper airway. However, it’s a good idea to talk to your dentist before using one, as they may move the position of your jaw or teeth.
If you have a medical condition such as central sleep apnea, a respiratory disorder, dental or jaw problems, or if you wear a dental device, you should consult with your doctor or dentist, as it may not be safe for you to wear a mouthguard. Anti-snoring mouthpieces sold over the counter aren’t intended to treat teeth grinding or sleep apnea, and they shouldn’t be considered a substitute for medical treatment of these conditions.
When choosing an anti-snoring mouthpiece, look for reputable brands that are cleared for treating snoring by the Food and Drug Administration.
The expected lifespan of an anti-snoring mouthpiece can range from two months up to three years, depending on the materials and how well it’s maintained. You may wear down your mouthpiece sooner if you grind your teeth or clench your jaw.
If you have symptoms of obstructive sleep apnea such as daytime sleepiness or waking up gasping for air, you should not attempt to self-treat the condition with an anti-snoring mouthpiece. Although these devices may help reduce snoring, which is a common symptom of OSA, it’s important to get proper medical treatment to avoid long-term health impacts.
Over-the-counter anti-snoring mouthpieces differ from oral appliances specifically designed to treat sleep apnea. The latter devices require a prescription following an OSA diagnosis.
Most mouthpieces can be gently cleaned with a soft toothbrush and a mild soap or nonabrasive toothpaste. Avoid using hot water. Check the user guide for specific instructions on cleaning solutions that are safe to use with your device.
You should clean the mouthpiece every time you take it out of your mouth, let it dry, and store it in its case. To keep your mouthpiece clean, always brush your teeth before inserting the device.
While anti-snoring mouthpieces can be an effective treatment option for snoring, many people find they can reduce snoring and also improve their overall health with lifestyle changes, such as losing weight and avoiding alcohol or sedatives before bed. Propping up the head of the bed with an adjustable base may also reduce snoring. Some people find that using a specially designed pillow, such as a wedge pillow, helps with snoring.
For snoring that worsens during allergy season, it may be helpful to use nasal sprays to clear your nasal passages. Speak with your doctor if you have concerns about your snoring, as it may be a sign of a more serious health condition.
Get Your Sleep Questions Answered Live on 4/30
Have questions about sleep? Get all your sleep-related questions answered in a Live Q&A on YouTube with renowned sleep expert Dr. Michael Breus at 5 p.m. PST/8 p.m. EST.